Lyophilization
QUALITY BY DESIGN IN PHARMACEUTICAL PRODUCTION USING A DIGITAL TWIN
DEVELOPMENT OF A DIGITAL TWIN FOR A LARGE-SCALE LYOPHILIZERS: MODELING, SIMULATION AND PROCESS PREDICTION
PROJECT LEAD: Dr. Matej Zadravec
PROJECT DURATION: July 1, 2023 to June 30, 2026
Lyophilization is a process in which a substance is dried at lower temperatures than in conventional drying. In lyophilization, a solution of the substance, typically an aqueous solution, is frozen and the solvent is then removed via sublimation.
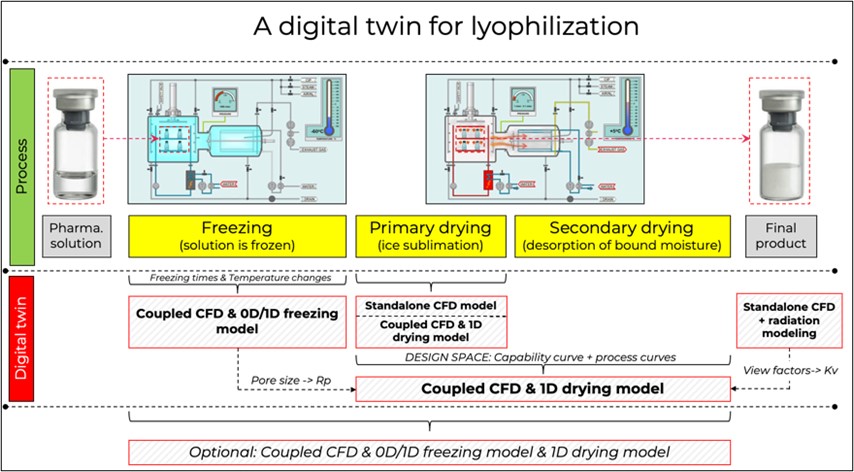
In the pharmaceutical industry, lyophilization is performed with target substances in small containers, i.e. vials, and consists of three main stages: the freezing stage and the primary and secondary drying stages. Sometimes there is also an annealing stage between the freezing and primary drying stages.
The goal of lyophilization is to produce a product that has a long shelf life and does not change its properties during rehydration. The ultimate goal of this project is to create a digital twin of the lyophilization process for a number of large scale lyophilizers. This goal can be accomplished by systematically developing a simulation model in a series of work packages.
MAIN OBJECTIVES
01
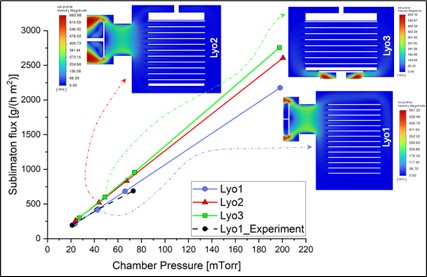
Establishing a method for calculating equipment capability for large scale lyophilizers.
02
Predicting the lyophilization equipment capabilities using CFD and Compact model.
03
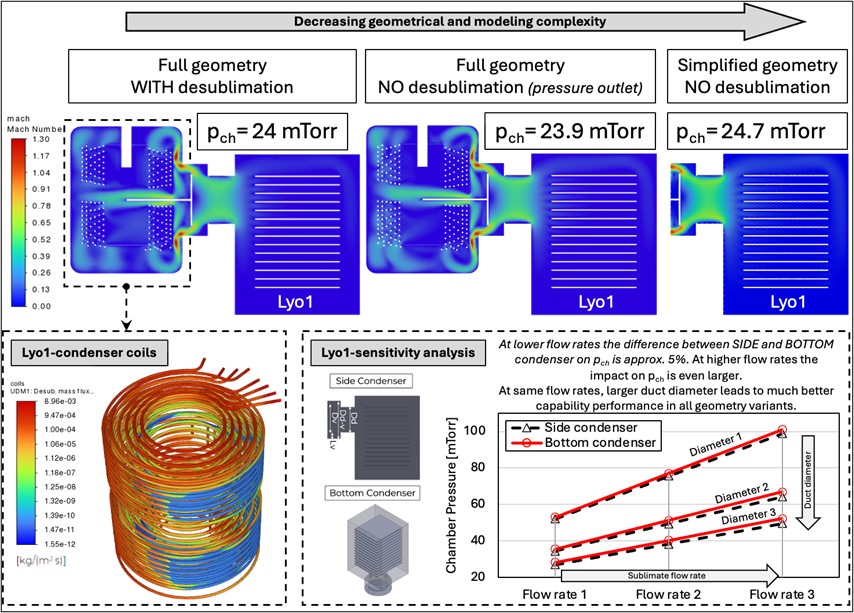
Prediction of mass transfer resistance coefficient by use of modeling.
04
Coupled sublimation and desublimation modeling in lyophilizers.
05
Coupled freezing, primary and secondary drying stage in lyophilizers.
06
Digital twin of small-scale and large-scale lyophilizer (Equipment and vial scale coupled model).
Learn more about our other projects: