Automated Continuous Manufacturing of Highly Potent APIs
BETTER PRODUCTS THROUGH AUTOMATION
DEVELOPMENT OF A CONCEPT FOR AN AUTOMATED CONTINUOUS MANUFACTURING LINE FOR HIGHLY POTENT APIS
PROJECT LEAD: Dr. Manuel Zettl
PROJECT DURATION: November 1, 2021 to October 31, 2024
For the production of highly potent (HP) active pharmaceutical ingredients (APIs), a concept for automating a continuous manufacturing line, encompassing API synthesis and downstream processing (e.g. crystallization, filtering, and drying), will be developed. The line will be modularly planned, allowing flexibility to accommodate various combinations of unit operations, and it will be made up of continuously, and batch-wise (where needed) operated units.
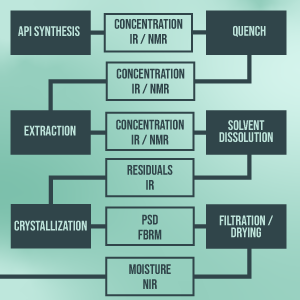
Integrating modern PAT techniques in the lab-scale processes will generate real-time process data for implementing a cutting-edge model-based control concept of all evaluated unit operations and combinations thereof. Simulation approaches will be used to implement and test the developed control strategy. This figure shows a process overview comprising possible unit operations and potential PAT sensors.
We will consider continuous HP API production requirements as we create innovative concepts for cleaning, cleaning validation, and loss of primary containment. To ensure a smooth transition toward commercial deployment, the project will investigate regulatory demands at each development stage. This will include developing a workflow toward regulatory approval of this innovative production setup.
MAIN OBJECTIVES